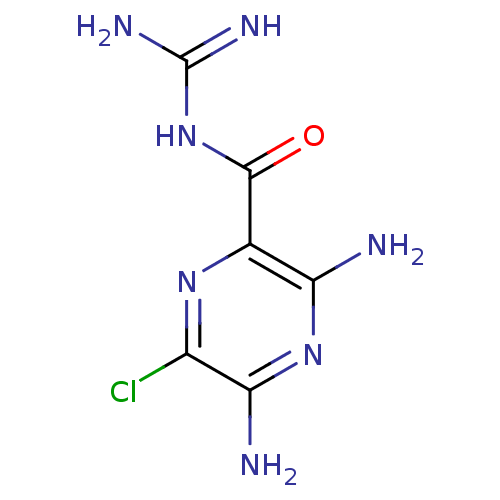
BDBM16173 3,5-diamino-6-chloro-N-(diaminomethylene)pyrazinamide;hydrochloride::3,5-diamino-N-carbamimidoyl-6-chloropyrazine-2-carboxamide::Amiloride::Amipramidin::CHEMBL945
SMILES NC(=N)NC(=O)c1nc(Cl)c(N)nc1N
InChI Key InChIKey=XSDQTOBWRPYKKA-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 16173
Found 4 hits for monomerid = 16173
Affinity DataKi: 3.28E+3nMAssay Description:Binding affinity for HA-tagged mutant human Adenosine A2A receptor (H250N) using [3H]-CGS-21,680 as radioligand expressed in COS-7 cellsMore data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Rattus norvegicus (rat))
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
Affinity DataKi: 1.16E+4nMAssay Description:Binding affinity for HA-tagged mutant human Adenosine A2A receptor (V84L), using [3H]CGS-21680 as radioligand expressed in COS-7 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.20E+4nMAssay Description:Binding affinity for HA-tagged wild type human Adenosine A2A receptor (WT) using [3H]CGS-21680 as radioligand expressed in COS-7 cellsMore data for this Ligand-Target Pair